How Medical Affairs Impact the Pharmaceutical Industry
Despite being crucial for scientific communication between companies and industry leaders, the growing responsibility of medical affairs has contributed to various challenges.

Source: Getty Images
- Nearly every medical device or pharmaceutical company has a medical affairs team responsible for gathering scientific data and communicating it with leaders in the pharmaceutical industry. However, a growing set of requirements for medical affairs departments has led to challenges for these companies.
When assessing the impact of medical affairs departments, it’s critical to define their role, identify potential challenges, and coordinate solutions to establish and maintain these vital components of the healthcare industry.
What Are Medical Affairs Departments?
The Accreditation Council for Medical Affairs (ACMA) defines medical affairs as a component of life sciences and pharmaceutical companies focusing on communication between the company or industry leaders and medical professionals or patients. Communication can be done through one or more of the following channels:
- Standard response documents
- Presentations
- Educational collateral
- Publications
- Field interactions
The ACMA notes that pharmaceutical and medical products companies developed medical affairs departments to answer physicians’ questions about clinical trial data and drug information. Since its inception, medical affairs teams have evolved to facilitate communication beyond commercial oversight.
Today, medical affairs departments support regulatory affairs, sales, and marketing.
READ MORE: Addressing Clinical Trial Challenges and High Drug Development Costs
While not required by law, many government agencies and regulatory organizations, including the United States Department of Health and Human Services and the FDA, strongly recommend having a medical affairs team to facilitate practical and lawful communication between pharmaceutical companies and healthcare providers (HCPs).
Medical affairs teams participate in multiple components of the healthcare industry and communication between providers and industry leaders. While their responsibilities may change depending on their exact role and the company, most medical affairs teams for pharmaceutical companies provide information on publications, off-label use, and safety to potential healthcare prescribers when a new drug comes to market.
For example, after a drug goes through clinical trials and is ready for market access, medical affairs will communicate the real-world applications of the medicine to potential healthcare prescribers using clinical and scientific information.
Additional responsibilities of medical affairs teams may include conducting rough clinical trials that are non-registered, analyzing health economics, product development through a scientific strategy, internal assessments for clinical trial proposals, outlining a plan for publications, developing educational tools, and reporting the latest news and insights to the rest of the team.
Internally, medical affairs bring together the research and commercial sides of the business by analyzing and translating important facts about therapeutics and drugs into language that can be better understood by a company whose expertise is not scientific.
READ MORE: Understanding the Pharmaceutical Drug Development Life Cycle
According to an insight report published by Deloitte, four primary components of medical affairs drive excellence and growth for the pharmaceutical companies that adopt them:
- Patient-centric development strategy
- Market-driven launch model
- Medically led stakeholder engagement
- Custodians of the benefit–risk ratio
Who Is on the Medical Affairs Team?
The medical affairs team is staffed with exceptionally well-educated personnel with advanced degrees, including PharmDs, which often direct the department.
Beyond the medical director, medical science liaisons (MSLs) are vital members of the medical affairs team. Roughly 75% of the MSLs have a PharmD or PhD; however, some may have a master’s degree, MD, or other medical education. These advanced degrees support their ability to provide unbiased medical information, generate evidence from studies, clinical trials, and clinical audits, and gather insights from members of the clinical trial process.
Other team members include the following:
- Medical advisors
- Scientific managers
- Clinical trial liaisons
- Medical lead
- Medical information officer
- Health economics and outcomes researcher
- Medical affairs trainers
Below is a Viseven graphic displaying key medical affairs community members.
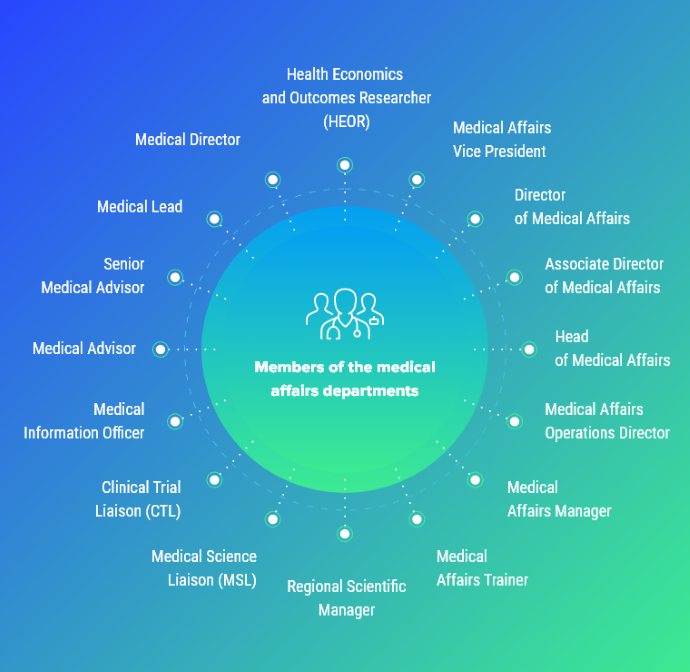
Source: Viseven
READ MORE: Comparing Small Molecule and Biologics Drug Development Challenges
Critical Issues for Medical Affairs Departments
Despite the importance of medical affairs teams and leaders, these departments’ challenges continue to increase. Medical affairs professionals have held more regulatory responsibilities in recent years as the protocols have evolved.
As external stakeholders, including members of the medical community and healthcare system, advocate for unbiased scientific knowledge and real-world evidence to support clinical decision-making, the role of medical affairs teams has adapted to fit the new framework.
The increased use of MSLs or regional medical liaisons to engage in medical communications with healthcare providers requires comprehensive policies and training of sales and marketing employees.
One primary challenge facing medical affairs teams is the varying demands of specific stakeholders. There is an increased demand for MSLs in the pharma industry; however, a reduced number of US sales and marketing employees.
Medical affairs function to provide unbiased information to medical teams about products; however, if pharma companies do not hire sales and marketing teams, MSLs may find themselves in an ethical bind if they market products instead of providing unbiased medical information.
According to a 2023 Medical Affairs Roundtable Discussion hosted by Charles River Associates (CRA), one of the biggest challenges medical affairs faces is a lack of awareness of its impacts. While this component of the healthcare sector is vital for evidence-based ethical practices, many people are unaware of its effects.
CRA recommends that pharmaceutical and medical device organizations incorporate language that relays value into their goals and key messages for medical affairs teams.
Another challenging factor of medical affairs is determining the best methods of scientific communication. Healthcare professionals and key opinion leaders (KOLs) are adaptive and innovative in collecting information, requiring medical affairs departments to consider factors including the generational divide among HCPs, increased interactions for HCPs, and the need for medical engagement.
The Evolving Role of Medical Affairs
As the healthcare industry has evolved, medical affairs teams have had to transform to keep up. In a report by McKinsey, the company points to multiple strategies to continue the transformation of medical affairs programs and maintain their relevance.
The company divides key activities projected for medical affairs teams in 2025 into four categories: innovative evidence generation, accelerated treatment access, transformed and personalized medical engagement, and advanced internal medical leadership.
The organization anticipates that by 2025, medical affairs teams will be responsible for more innovative evidence generation through planning, advanced analytics, optimized clinical trials, collaborative research, and publications.
One recommendation includes assessing the digital landscape of healthcare and leveraging new digital tools for scientific communication. The report explains that “social media and digital have supported consumers through the dissemination of knowledge and raised expectations; in healthcare, this has brought demands from patients, healthcare practitioners (HCPs), and medical leaders at payers, health systems, and elsewhere, as they want more complex but digestible and relevant information at their fingertips.”
An additional role by medical affairs companies in 2025 is to accelerate access to treatments by focusing on valuable data generation, value-based field medical communications, and an increased number of patient-facing activities.
These teams will be expected to improve stakeholder engagement with management strategies, automation and digitization of medical information, and collaborative medical education efforts.
Finally, medical affairs teams are expected to improve medical strategy and produce talented medical and pharmaceutical industry leaders.
Overall, medical affairs are a vital and evolving component of medicine that requires a holistic and unbiased view of medical information, scientific data, patient needs, and stakeholder goals.
