What to Know About the 2023 Eyedrop Recalls, Understanding the Risks
With multiple recalls reported this year, clinicians and patients must understand the risks associated with the 2023 eyedrop recalls. Here’s what to know.

Source: Getty Images
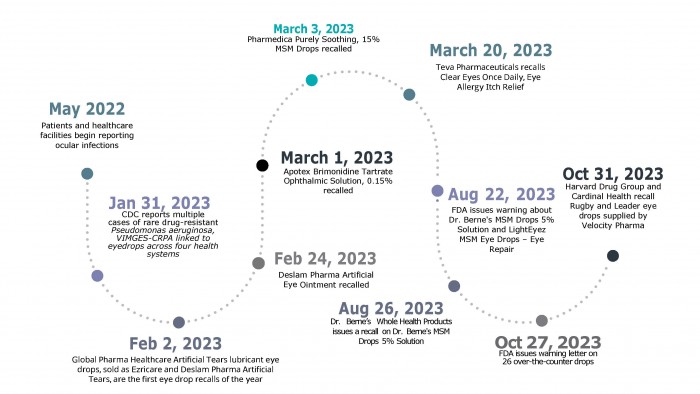
- On February 2, 2023, the first of three eyedrops in the United States were recalled voluntarily after multiple reports of illness associated with the drops were reported to the CDC. Since then, two additional eye drops in the US have been recalled. More recently, in October 2023, 26 other eyedrops from major brands, including CVS and Velocity Pharma, were recalled.
Although the FDA has released information on the recalls through its Recalls, Withdrawals, and Safety Alerts program, clinicians, health officials, the public, and manufacturers need a deeper dive into what went wrong to understand the risks better and prevent recalls moving forward.
Three Initial Eye Drop Recalls
PharmaNewsIntelligence first reported on the artificial tears recall in early February. Within weeks, in early March, the FDA and manufacturing companies had recalled two additional eyedrops, begging the question: why have so many recalls happened? Before answering why, it is essential to understand the scope and impact of these recalls. The three recalled products included the following brands of eyedrops: Global Pharma Healthcare, Apotex Corp, and Pharmedica USA LLC.
Global Pharma Healthcare
Global Pharma Healthcare drops were the first eye drops linked to Pseudomonas aeruginosa, a bacterium commonly found in soil and water. On January 31, 2023, the CDC reported multiple cases of a rare, dangerous, and drug-resistant P. aeruginosa, VIM-GES-CRPA.
At the time, CDC investigators identified 55 cases across 12 states in the US. Additionally, five patients reported temporary or permanent vision loss, and one case of infection was fatal. The analysis by the CDC said that these cases were linked to four healthcare facilities, and the patient infections began as early as May 2022.
In-depth patient histories and inquisition led the CDC to identify artificial eye drops as the common denominator across these infections. Although the brand varied, most patients with confirmed conditions were using Ezricare Artificial Tears. The company championed these eye drops as preservative-free, over-the-counter lubricating drops.
Based on this data, Global Pharma Healthcare issued a voluntary recall notice naming its Artificial Tears Lubricant Eye Drops, which EzriCare LLC and Delsam Pharma distribute. Despite a voluntary recall, the FDA placed the company, based in India, on an import alert due to violations of current good manufacturing practices (CGMPs).
Since the initial announcement, the CDC has identified 13 additional cases of infection associated with the Ezricare and Delsam Pharma Artificial Tears, for a total of 68 cases across 16 states. As of March 14, 2023, the CDC has reported eight cases of vision loss, with four patients having their eyeballs surgically removed (enucleation).
Beyond recalls on artificial tears, the FDA recommended that Global Pharma Healthcare issue a recall on Delsam Pharma Artificial Eye Ointment used to relieve dry eyes on February 24, 2023. According to the FDA announcement, the company has reported possible microbial contamination and package leaking.
Apotex Corp
Just weeks later, Apotex Corp issued a voluntary recall on their Brimonidine Tartrate Ophthalmic Solution, citing a potential lack of sterility as its leading cause. The α-adrenergic receptor agonist is prescribed to reduce intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
The six affected lots were distributed between April 5, 2022, and February 22, 2023. Apotex, headquartered in Weston, Florida, identified cracks in the solution bottle caps. Because of the damages, the manufacturer is concerned about sterility.
Pharmedica USA LLC
Two days after the Apotex recall, the FDA released information on another recall by Pharmedica USA LLC, based in Arizona, on two lots of Purely Soothing, 15% MSM drops. The product is an anti-inflammatory medication for those with ocular irritation or swelling.
While the company issued the recall due to potential sterility issues, the organization has not received any reports of infection or adverse events related to the product. Pharmedica says the recall is just out of an abundance of caution.
Even More Recalls
Despite a brief lull in eye drop recalls after those three initial drops, the market faces supply chain difficulties as more eye drops are recalled.
For example, on March 20, 2023, Teva Pharmaceuticals recalled five lots of its Clear Eyes Once Daily Eye Allergy Itch Relief drops, citing unspecified impurities.
Months later, on August 22, 2023, the FDA warned about two additional drops due to bacterial and fungal contaminations. The FDA warning noted that Dr. Berne's MSM Drops 5% Solution may be contaminated with Bacillus spp. and Exophiala sp. Meanwhile, the LightEyez MSM Eye Drops – Eye Repair drops were infected with Pseudomonas spp., Mycobacterium spp., Mycolicibacterium spp., and Methylorubrum spp.
Although LightEyez did not respond to the public warning, Dr. Berne’s Whole Health voluntarily recalled their product on August 26, 2023.
More notably, on October 27, 2023, the FDA warned consumers about 26 over-the-counter eye drops with potential sterility issues that can cause eye infections, leading to partial or total vision loss.
The warning statement mentioned multiple major brands, including CVS Health, Cardinal Health brands like Leader and Rugby, Rite Aid, Target Up & Up, and Velocity Pharma. The administration updated the warning letter three days later, adding Walmart to the list.
While a more comprehensive list of products can be found on the FDA’s website, below are some drops listed in the statement:
- CVS Health Lubricant Eye Drops 15 mL (Carboxymethylcellulose Sodium Eye Drops 0.5% w/v), single and twin packs
- CVS Health Multi-Action relief Drops 15 mL (Polyvinyl Alcohol 0.5% w/v & Povidone 0.6% w/v & Tetrahydrozoline Hydrochloride 0.05% Eye Drops)
- Rugby Lubricating Tears Eye Drops 15 mL (Hypromellose 2910-0.3% w/v & Dextran 70- 0.1% Eye Drops)
- Rugby Polyvinyl Alcohol 1.4% Lubricating Eye Drops 15 mL (Polyvinyl Alcohol Eye Drops 1.4% w/v)
- Leader Dry Eye Relief 10 mL (Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops)
- Rite Aid Gentle Lubricant Gel Eye Drops 15 mL (Hypromellose 0.3%, Glycerin 0.2%, Dextran 70 0.1% Eye Drops)
- Target Up&Up Extreme Relief Dry Eye 15 mL (Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops)
- Velocity Pharma LLC Lubricant Eye Drop 10 mL (Propylene Glycol Eye Drops 0.6% w/v)
- Walmart Equate Hydration PF Lubricant Eye Drops 10 mL (Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops)
After the warning letters were released, multiple companies issued voluntary product recalls. For example, on October 31, 2023, the Harvard Drug Group LLC, which manufactures the Rugby eyedrops, published a recall alongside a risk statement warning patients that eye infections could result in vision loss.
On the same day, Cardinal Health released a recall of the Leader eye drops that Velocity Pharma supplied. In fact, an article from the Washington Post suggests that the retailers have all supplied their drops from Velocity Pharma, which has yet to respond.
The FDA emphasized that contamination in eye drops or other ocular products can have an incredibly harmful effect on consumers. They circumvent some natural biological defenses because they are inserted directly into the eye.
Below is a timeline that reflects the progression of these recalls.
Source: Xtelligent Healthcare Media
Ocular Infections
According to the Illinois Eye Center, ocular infections, called uveitis, are common in people between 20 and 60 years old. Uveitis is a broad term for many diseases, including blepharitis, cellulitis, corneal ulcers, keratitis, conjunctivitis, styes, and trachoma, that can cause eye swelling and deteriorating eye tissues.
There are multiple symptoms associated with eye infections, including the following:
- Chronic redness of the eyes
- Persistent itching
- Flaking eyelids
- Eye discomfort
- Blurry vision
- Watery eyes
- Discharge
- Eye pain
- Swelling of the eyes and eyelids
The American Academy of Ophthalmology notes that bacterial keratitis, an infection in the cornea, can develop quickly, causing blindness. The most common types of bacteria linked to keratitis are Staphylococcus aureus and Pseudomonas aeruginosa.
Pseudomonas aeruginosa
StatPearls defines P. aeruginosa as a “gram-negative, aerobic, non-spore-forming rod that is capable of causing a variety of infections in both immunocompetent and immunocompromised hosts.” The bacteria are commonly found in freshwater.
According to the CDC, symptoms of this bacterial infection in the eye may include yellow, green, or clear discharge, eye irritation or pain, redness, foreign body sensations, light sensitivity, or blurred vision.
Because this infection is widespread in healthcare settings and many clinicians prescribe antibiotics, the bacteria have evolved to have drug-resistant strains. The most recent drug-resistant strain, VIM-GES-CRPA, was identified during the eye drop recall.
Recently, JAMA Ophthalmology published a case study on a 72-year-old patient infected with drug-resistant bacteria after using one of the recalled eye drops, namely, Ezricare. The patient reported blindness in her left eye for one week. Researchers in the study noted that for the left eye, “physical examination was notable for visual acuity of light perception (LP) and a hypopyon filling the anterior chamber with an epithelial defect involving most of the cornea.” Meanwhile, the right eye was unaffected.
Although the patient was treated with multiple antibiotics, the patient developed a corneal ulcer that needed to be treated.
What Caused the Eyedrop Recalls?
As the eyedrop recalls swept the news back-to-back, clinicians, healthcare providers, and patients were all left wondering why so many recalls existed. Despite speculation, there have been no confirmed causes that explain the contamination for all of the recalls.
However, looking at the defects mentioned and manufacturing protocols, healthcare professionals may be able to extrapolate information on the cause of the recalls and how to prevent them down the line.
As PharmaNewsIntelligence mentioned in previous articles, the sequential recalls indicate supply chain and manufacturing issues.
One of the manufacturers, Global Pharma Healthcare, received a notice for violating CGMPs, indicating that the company did not follow manufacturing protocols advised by the FDA. A deep dive into the manufacturer by the FDA identified multiple violations, including 19 breaches of mandatory quality control tests that were disregarded or irregular.
Beyond that, the evaluation of the manufacturing site found that dirty equipment or contaminated containers were used in clean room environments. Finally, the report found that the tools used by Global Pharma to filter the eye products were not effective.
While the FDA does not clarify which practices have not been followed, data from the CDC investigation may imply errors beyond the packaging errors cited in other recalls.
The CDC’s investigation into the spread of infection found bacteria in two bottles of Ezricare Artificial Tears. The patients whose bottles were tested had not reported illness or tested positive for the bacteria, indicating that the bacteria were independent of patient error and use.
In addition to the reported manufacturing errors, there are other theories about the expanded rate of eye drop recalls.
One theory is that the lack of preservatives in the Global Pharma eye drops made them more susceptible to contamination. Most multi-dose eyedrop vials contain a preservative called benzalkonium chloride (BAK).
According to a publication in Clinical Ophthalmology, BAK is a potent antimicrobial agent in many topical ophthalmic drops that can prevent contamination. Despite its benefits, high doses of BAK in the long term can cause damage and negative symptoms such as burning, dry eyes, and itching in 30–50% of regular users.
Continued and regular use of eye drops to treat ophthalmic conditions may increase the prevalence of severity of symptoms. More severe symptoms may include lower corneal sensitivity, decreased nerve sensitivity, and hypoesthesia.
The link between BAK overexposure and ocular symptoms has driven many people, including ophthalmologists, to preservative-free options. Although BAK-containing eye drops are not ideal for some patients, there are alternative options that can minimize the risk of contamination and recall.
The AOA explained, “It’s important to note that eye drops are safe when manufactured and used properly. The AOA recommends all patients consult with their local AOA optometrist before purchasing eye drops to ensure an appropriate treatment plan is in place. When talking with a doctor of optometry, it’s also important to share any prescribed and over-the-counter medications currently in use to avoid health complications.”
First, if a patient or provider is adamant about a preservative-free approach, they should opt for single-use eye drop vials. Since the patient does not close and reopen the bottle as they do with a multi-use vial, there is a lower risk of contamination.
Alternatively, other preservative options can be used for eye drops. While they are not an industry standard, other alternatives may have a less detrimental impact with prolonged use.
Beyond contamination and a lack of preservatives, errors in sterility while manufacturing may also lead to recalls. Even though companies may not have reported adverse effects, it is good practice to issue recall products once a sterility error has been identified.
Finally, manufacturers are advised to conduct robust material testing to address containment errors. Quality control protocols and post-market evaluations may also help detect errors early on.
Editor's Note: This article has been updated to reflect new recalls and provide more context.
