How Is Real-World Evidence Impacting Drug Discovery, Development
The pharmaceutical industry is turning to real-world evidence to improve drug discovery and development, and new FDA guidance should see that trend continue.

Source: Getty Images
- An estimated $2 billion represents the total amount spent on drug development. In support of its call for expanding the use of RWE in regulatory and value-based payment decision-making for drugs and biologics, the Bipartisan Policy Center highlighted the fact that $1.5 billion of that amount of goes toward clinical trials:
“Researchers in the United States have conducted clinical trials for FDA submissions for regulatory approval in essentially the same way for more than 50 years,” the authors wrote. “Such studies often involve a relatively homogeneous study population with the goal of attributing an outcome solely to the treatment, but the real world of patients is highly heterogeneous and unforeseen events — both positive and negative — can often occur when that treatment is used in real-world settings."
The pharmaceutical industry has heard the message, which explains the motivation behind drug companies looking to get more upstream of their major cost centers and determine the safety and effectiveness of their products more efficiently and cheaply.
Close to the start of the current decade, the pharmaceutical industry began to take a serious interest in real-world evidence.
In 2018, researchers at McKinsey & Company observed that pharmaceutical companies have gone through two generations of RWE use, with signs pointing to greater adoption in the future.
In Generation 1 (circa 2011), pharma mainly relied on RWE to focus on safety and post-market. Meanwhile, Generation II (2012–2015) saw the integration of RWE into the complete life cycle of pharma products.
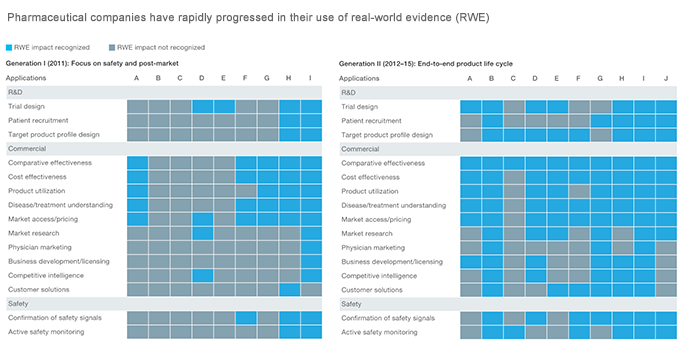
Source: McKinsey & Company
Adding fuel to the push for greater use of RWE, the Food and Drug Administration in 2018 released a long-awaited framework laying out the Real-World Evidence Program and its goal to use real-world evidence to support regulatory decision-making, such as approval for new indications for approved drugs.
So what is real-world evidence?
The FDA framework provides two important definitions:
Real-world data (RWD): data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources
Real-world evidence (RWE): clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD.
The framework lists numerous forms of RWD. More illustrative is McKinsey & Company’s definitions of the four major categories of real-world data.
First is clinical data:
These are patient-level data pulled from electronic medical records (EMR) and patient registries that describe how patients are treated in the real world. They include lab values, diagnoses, notes, and other information from healthcare visits with physicians and other care providers. With more data from hospitals and entire health systems becoming digitized and more easily integrated across institutions, the power of these particularly rich datasets (for example, larger sample sizes, easier comparisons across systems) is increasing.
Second is administrative/claims data:
Detailed patient-level data is also collected for non-clinical purposes, primarily for billing by providers to insurers and other payors, which can include diagnoses, services provided, costs, and other data required for the reimbursement of healthcare services. Other more administrative sources of data can also include data collected for tracking purposes, such as patient or population surveys.
The third is patient-generated/reported data:
This category covers individual data describing the patient’s experience and is typically both collected and shared/reported by the patient. Today this source of data is less prevalent than others but will likely expand due to the increased use of wearable devices that automate data collection and sharing. Online communities such as PatientsLikeMe encourage and enable sharing of patient-generated data with peers and investigators.
And the fourth and final (for the time being) are non-traditional, health-related digital data sources:
As digital becomes increasingly prevalent in our lives, new sources of patient-level health data are emerging. These span social media posts that have a rich trove of information, especially health-focused social media sites like Sage Bionetworks. Project Data Sphere is a pharmaceutical industry-sponsored platform to share, integrate, and analyze phase III comparator arm data from cancer trials to accelerate research.
Whereas RWD refers to data points and sources, RWE refers specifically to the “clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD.”
With interoperability still proving an obstacle to gathering RWE, the pharmaceutical industry must work closely with business partners to ensure the sharing of robust information. But with regulators keen on driving down healthcare costs while improving healthcare quality through the integration of RWE, drug developers must find viable pathways to RWD to translate into RWE.
